Meet your laboratory needs today and in the future with our broad and expanding menu of assays for a range of disciplines, including molecular testing.
Broad assay menu
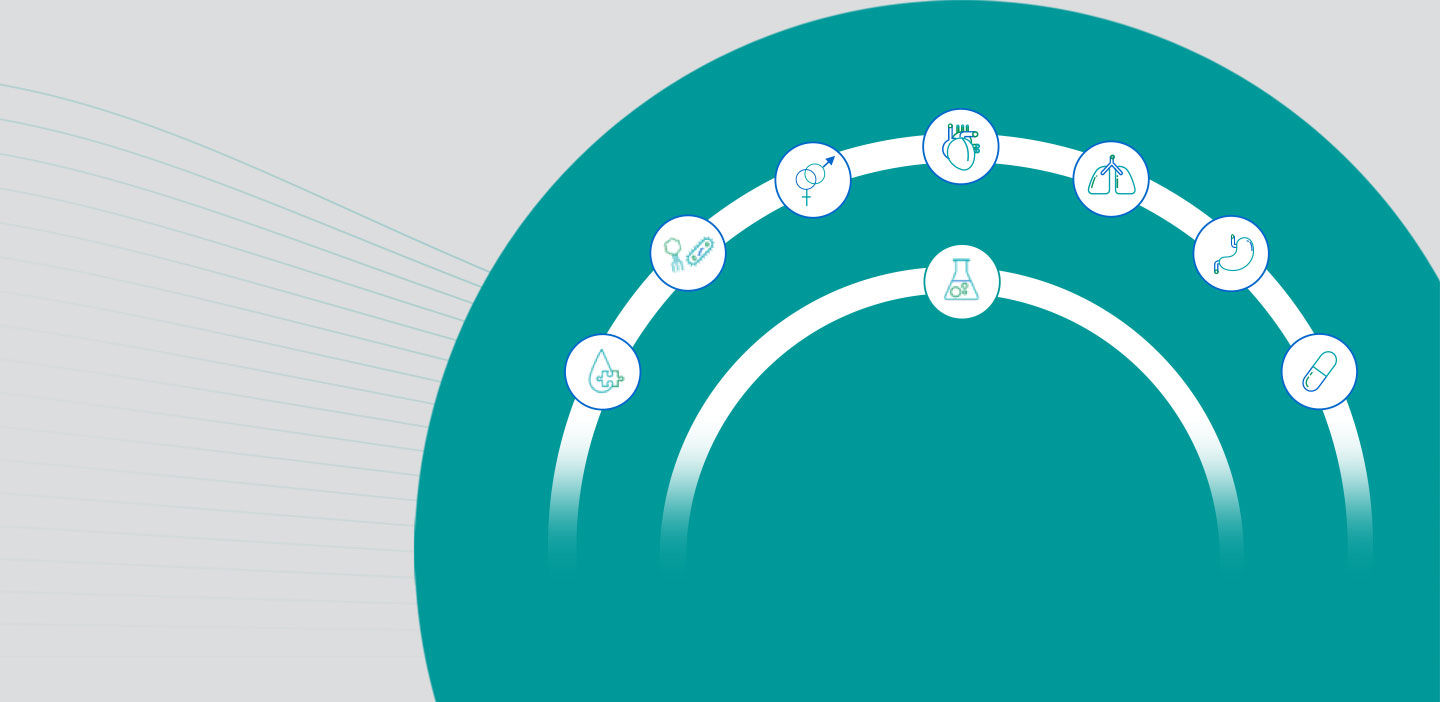
The right menu of assays consolidated on a single platform
Meet today's demands, explore tomorrow's possibilities
Every Roche assay is backed up by extensive experience and expertise in test design and optimization. Results on Roche instruments are also shown to be standardized across all platforms.1-4 This consistent excellence has made Roche the preferred diagnostic partner in clinical trials.5-6
The Molecular Work Area brings this demonstrated excellence into your laboratory by consolidating the right menu of assays onto a single platform, including a dedicated open channel for other assays that could benefit from consolidation.
With a shared reagent concept and standardized user experience, the Molecular Work Area is performing up to 10 million tests per month across 1,500+ systems globally, including 50 sites with integrated pre-analytical automation.
Want to know more?
Get exclusive insights and information from real labs
Deliver high medical value backed by a broad menu and consistent performance
Roche assays are developed according to WHO standards. They deliver consistent quality and collectively set the standard of performance across broad range of testing needs.
The Molecular Work Area has assays available for donor screening, infectious diseases and respiratory infections, sexual health, and transplant-related infections
Bringing IVD workflow gains to lab developed testing
The cobas omni Utility Channel on the cobas® 5800/6800/8800 Systems enables a broader testing menu by consolidating open channel assays with Roche in vitro diagnostics (IVD) assays on a single platform. Open channel assays may include IVD assays, as well as routine lab developed tests (LDTs). The ability to consolidate and automate a wider array of testing on a single platform helps to increase operational efficiency, maximize laboratory space and minimize capital investment.
Expanding coverage and extending confidence with advanced approaches to testing
Dual Target Technology
The rapidly mutating HIV-1 virus can continually evade quantification by a single target viral load assay. Roche's innovative dual target HIV-1 assay measures two unique regions of the HIV-1 genome, which are not subject to selective drug pressure. With this approach, drug-induced mutations should not impact the assay's ability to detect and quantify the virus accurately.
As more accurate results can drive better decisions, we expanded the application of this technology to have a greater impact on patient lives. Incorporating it into our recently-announced tests for EBV and BKV will help guard against the risk of sequence variations that may be present in the viruses. These tests will work to ensure quantitative accuracy when screening transplant patients at risk of complications caused by the viruses.
Dual Probe Approach
Our quantitative HCV RNA assay is based on a novel dual probe approach with an optimized automated extraction and amplification procedure. This test ensures highly sensitive detection of HCV RNA across different HCV genotypes with accurate and reproducible results at low viral loads. With improved testing, optimized treatment is one step closer—as is putting a stop to the spread of HCV.
Like what you read? Share it:
True consolidation—only from Roche
Roche is the established partner labs need to meet current and future demands—from defining clinical cut-off points in the wider marketplace to mitigating infection risk and promoting the standardization of testing.
References:
- Vermehren J, Stelzl E, Maasoumy B, et al. Multicenter Comparison Study of both Analytical and Clinical Performance across Four Roche Hepatitis C Virus RNA Assays Utilizing Different Platforms. J Clin Microbiol. 2017;55(4):1131-1139.
- Maasoumy B, Bremer B, Lehmann P, et al. Commutability and concordance of four hepatitis B virus DNA assays in an international multicenter study. Therap Adv Gastroenterol. 2017;10(8):609-618.
- Adams P, Vancutsem E, Nicolaizeau C, et al. Multicenter evaluation of the cobas® HIV-1 quantitative nucleic acid test for use on the cobas® 4800 system for the quantification of HIV-1 plasma viral load. J Clin Virol. 2019;114;43-49.
- Data on file with Roche.
- Data on file with Roche.
- Data on file with Roche.
